ONCT-534, our Dual-Action Androgen Receptor Inhibitor (DAARI) candidate, is a novel investigational, potentially first-in-class, orally bioavailable, dual-action androgen receptor (AR) inhibitor, designed to treat patients with metastatic castration-resistant prostate cancer and other androgen receptor-driven diseases. Based on preclinical studies, we believe ONCT-534 has the potential to be a novel treatment option for patients with advanced resistant prostate cancer. We licensed ONCT-534 and certain other DAARI program rights from the University of Tennessee Research Foundation, or UTRF, under an exclusive, worldwide license agreement.
Addressing Unmet Needs in Prostate Cancer
Prostate cancer is the second most diagnosed cancer and the fifth leading cause of cancer death among men worldwide. In 2019, in the United States, 224,733 new cases of prostate cancer were reported among men, and 31,636 men died of this cancer. Current therapeutic strategies for advanced castrate-resistant prostate cancer (CRPC) include androgen receptor inhibitors (ARi), such as enzalutamide (XTANDI®; Pfizer), apalutamide (ERLEADA®; Janssen), and darolutamide (NUBEQA®; Bayer) that target the ligand-binding domain (LBD) of the androgen receptor (AR), and abiraterone (ZYTIGA®; Janssen), an androgen biosynthesis inhibitor, that inhibits 17α-hydroxylase/C17,20-lyase. Although these drugs extend progression-free survival, approximately 30% of patients do not respond to these therapies, and the remaining 70% of patients often develop resistance in 1 to 2 years after treatment initiation. The most common reasons for resistance to AR inhibitors include AR gene amplification, mutations in the LBD, and the expression of AR splice variants that lack part or all of the LBD. Therefore, there is an unmet clinical need for treatment of patients with advanced CRPC who have resistance to AR inhibitors by virtue of alterations of the AR.
Novel Mechanism of Action
As a Dual-Action Androgen Receptor Inhibitor or DAARI, ONCT-534 has a potentially novel and unique mechanism of action: it interacts with both ends of the AR, the N terminal domain (NTD) and the LBD, inhibiting AR function and leading to AR protein degradation. We believe that this NTD binding is relevant to the activity of ONCT-534 against tumors expressing AR splice-variants by preventing AR activation. In this respect, ONCT-534 is designed to mechanistically differ from classical non-steroid antiandrogens that interfere with androgen synthesis, such as abiraterone, and to differ from current standard of care treatment options, such as enzalutamide, darolutamide, or apalutamide, that bind only to the LBD of the AR, which may explain their reduced efficacy in patients with AR-SV-expressing tumors, as these AR variants lack most or all of the LBD. We believe that the differentiated dual-action pharmacology of ONCT-534 has the potential to help men with prostate cancer that is resistant to current standard of care treatments by virtue of amplified AR, LBD mutations, or AR splice variants with loss of LBD. Importantly, ONCT-534 is also active in preclinical models of prostate cancer with a normal, unmutated AR.
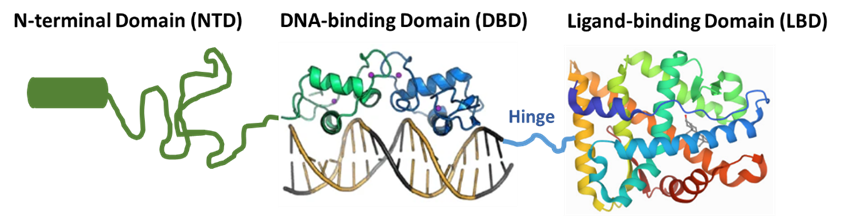
We believe our mechanism of action offers potential for DAARI therapeutic development in other AR-driven diseases, such as luminal AR-positive triple-negative breast cancer, or LAR-TNBC, as well as non-oncology rare disease indications, such as Spinal Bulbar Muscular Atrophy, or SBMA, also known as Kennedy’s Disease.
ONCT-534 Development in Prostate Cancer
In preclinical studies, ONCT-534 demonstrated antagonism and degradation of full-length AR, mutant LBD AR, and AR-splice variants. ONCT-534 additionally has shown strong in vivo activity in models of prostate cancer in both castrated and intact animals that are resistant to AR antagonists, such as enzalutamide, as detailed below.
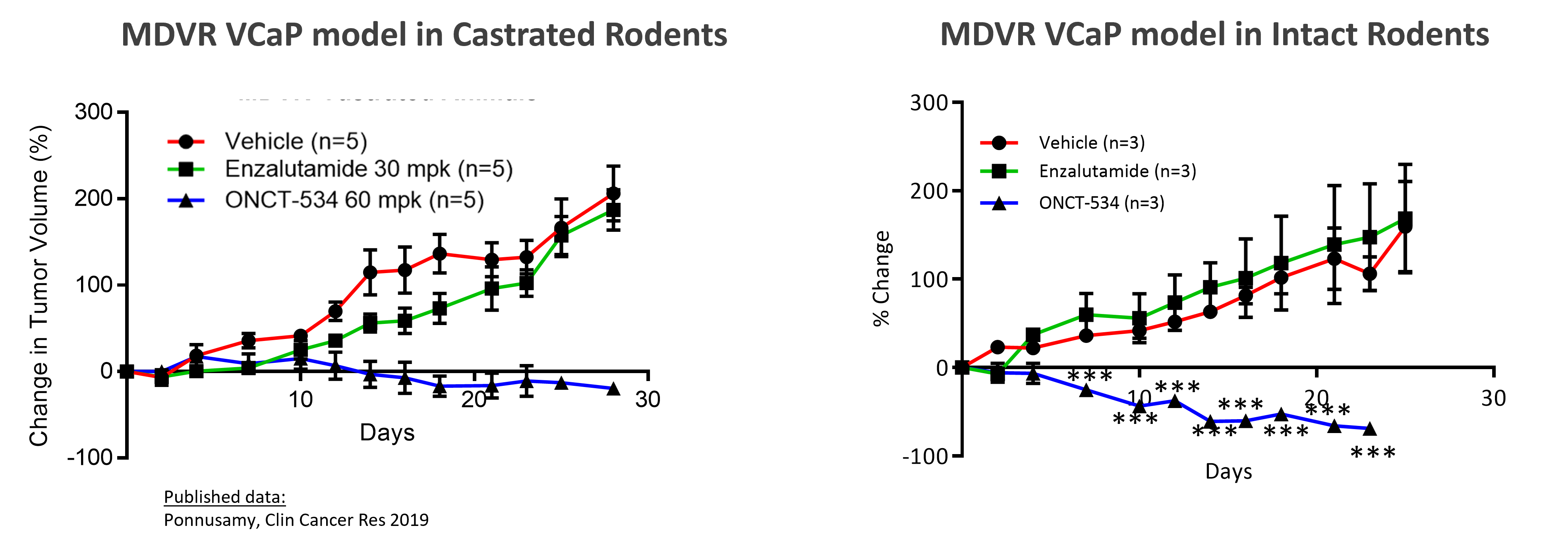
To assess the ability of ONCT-534 to treat enzalutamide-resistant prostate cancers, we conducted in vivo studies in an enzalutamide-resistant MDVR VCaP cell line xenograft model. This treatment resistance can be seen below for both castrated and intact animals, as tumors in mice dosed with enzalutamide grew at nearly the same rate as tumors in mice dosed only with the drug vehicle, a control similar to dosing with a placebo. Orally delivered ONCT-534 substantially inhibited tumor growth in these enzalutamide-resistant MDVR tumors.
ONCT-534 Exhibited Anti-tumor Activity in ENZA-resistant CRPC Preclinical Model
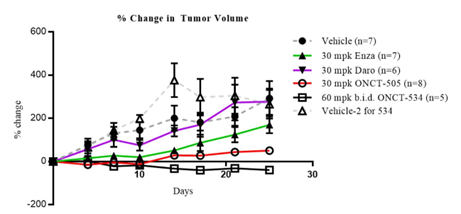
In a mouse xenograft model of human prostate cancer in intact animals, tumor growth of LnCAP human prostate cancer cells that overexpress AR (LnCAP-AR) was significantly inhibited by treatment with ONCT-534, as shown in the figure below.
DAARIs ONCT-534 and ONCT-505 Exhibited Anti-tumor Activity in AR-overexpressing Preclinical Model
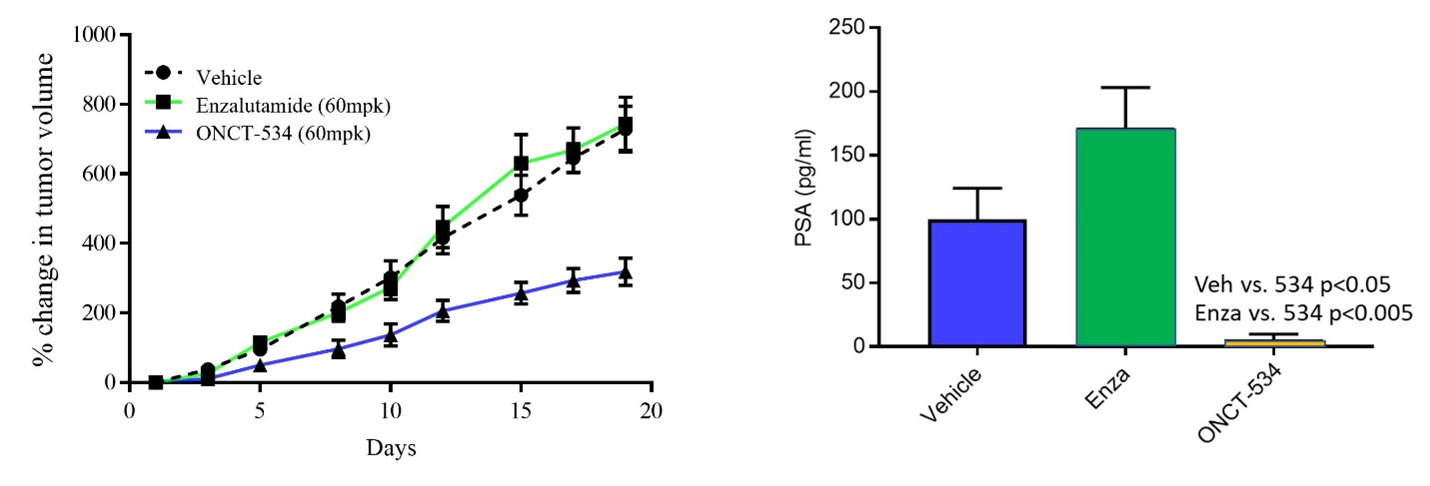
AR-V7 is a splice variant of AR that lacks the LBD and hinge region and is expressed in 22Rv1 cells, which are human prostate carcinoma epithelial cells derived from a xenograft that was serially propagated in mice after castration-induced regression and relapse of the parental, androgen-dependent xenograft. As shown below, enzalutamide is not efficacious against these tumors that lack the LBD. Treatment with ONCT-534 however, resulted in tumor growth inhibition as well as significant reduction in PSA in this model, demonstrating activity at the NTD.
ONCT-534 Exhibited Anti-tumor Activity in AR Splice Variant-expressing Preclinical Model
On September 12, 2024, Oncternal announced it was discontinuing its clinical trials and exploring strategic alternatives.

